6.12: Battery characteristics
- Page ID
- 21178
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The following battery characteristics must be taken into consideration when selecting a battery:
- Type
- Voltage
- Discharge curve
- Capacity
- Energy density
- Specific energy density
- Power density
- Temperature dependence
- Service life
- Physical requirements
- Charge/discharge cycle
- Cycle life
- Cost
- Ability to deep discharge
- Application requirements
Voltage
The theoretical standard cell voltage can be determined from the electrochemical series using Eo values:
Eo (cathodic) – Eo (anodic) = Eo (cell)
This is the standard theoretical voltage. The theoretical cell voltage is modified by the Nernst equation, which takes into account the non-standard state of the reacting component. The Nernstian potential will change with time either because of use or self-discharge by which the activity (or concentration) of the electro-active component in the cell is modified. Thus the nominal voltage is determined by the cell chemistry at any given point of time.
The actual voltage produce will always be lower than the theoretical voltage due to polarisation and the resistance losses (IR drop) of the battery and is dependent upon the load current and the internal impedance of the cell. These factors are dependent upon electrode kinetics and thus vary with temperature, state of charge, and with the age of the cell. The actual voltage appearing at the terminal needs to be sufficient for the intended application.
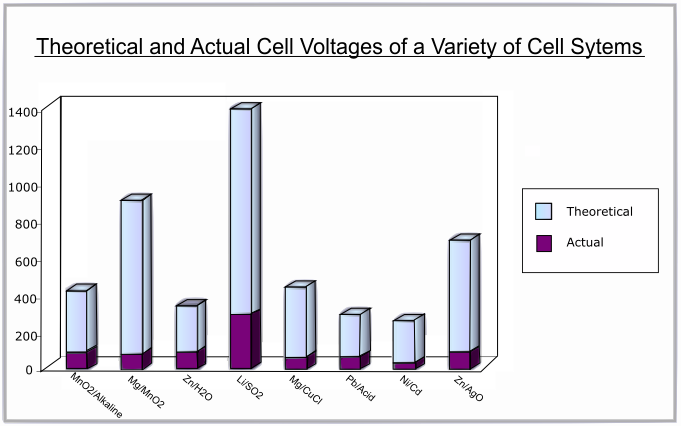
Typical values of voltage range from 1.2 V for a Ni/Cd battery to 3.7 V for a Li/ion battery.
The following graph shows the difference between the theoretical and actual voltages for various battery systems:
Discharge Curve
The discharge curve is a plot of voltage against percentage of capacity discharged. A flat discharge curve is desirable as this means that the voltage remains constant as the battery is used up.
Capacity
The theoretical capacity of a battery is the quantity of electricity involved in the electro-chemical reaction. It is denoted Q and is given by:
\[Q=x n F\]
where x = number of moles of reaction, n = number of electrons transferred per mole of reaction and F = Faraday's constant
The capacity is usually given in terms of mass, not the number of moles:
\[Q=\frac{n F}{M_{r}}\]
where Mr = Molecular Mass. This gives the capacity in units of Ampere-hours per gram (Ah/g).
In practice, the full battery capacity could never be realised, as there is a significant weight contribution from non-reactive components such as binders & conducting particles, separators & electrolytes and current collectors & substrates as well as packaging. Typical values range from 0.26 Ah/g for Pb to 26.59 Ah/g for H2.
Energy density
The energy density is the energy that can be derived peer unit volume of the weight of the cell.
Specific energy density
The specific energy density is the energy that can be derived per unit weight of the cell (or sometimes per unit weight of the active electrode material). It is the product of the specific capacity and the operating voltage in one full discharge cycle. Both the current and the voltage may vary within a discharge cycle and thus the specific energy derived is calculated by integrating the product of current and voltage over time. The discharge time is related to the maximum and minimum voltage threshold and is dependent upon the state of availability of the active materials and/or the avoidance of an irreversible state for a rechargeable battery.
Power density
The power density is the power that can be derived per unit weight of the cell (W/kg).
Temperature dependence
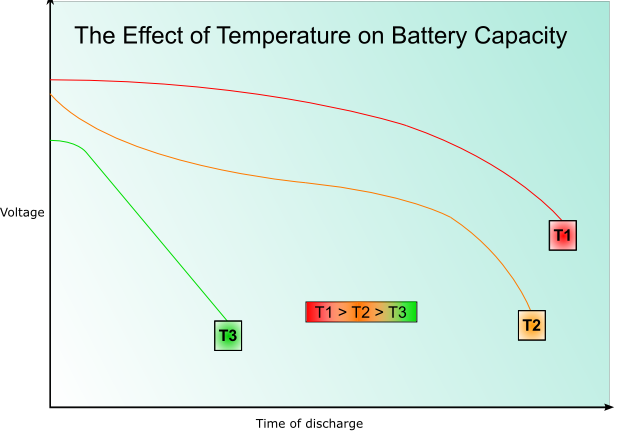
The rate of the reaction in the cell will be temperature dependant according to theories of kinetics. The internal resistance also varies with temperature; low temperatures give higher internal resistance. At very low temperatures the electrolyte may freeze giving a lower voltage as ion movement is impeded. At very high temperatures the chemicals may decompose, or there may be enough energy available to activate unwanted, reversible reactions, reducing the capacity.
The rate of decrease of voltage with increasing discharge will also be higher at lower temperatures, as will the capacity- this is illustrated by the following graph:
Service life
The battery cycle life for a rechargeable battery is defined as the number of charge/recharge cycles a secondary battery can perform before its capacity falls to 80% of what it originally was. This is typically between 500 and 1200 cycles.
The battery shelf life is the time a battery can be stored inactive before its capacity falls to 80%. The reduction in capacity with time is caused by the depletion of the active materials by undesired reactions within the cell.
Batteries can also be subjected to premature death by:
- Over-charging
- Over-discharging
- Short circuiting
- Drawing more current than it was designed to produce
- Subjecting to extreme temperatures
- Subjecting to physical shock or vibrations
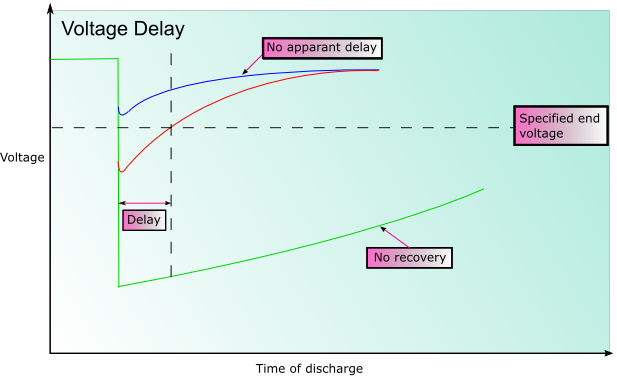
Voltage Delay
In some battery systems passivation may occur. Passivation is the process by which the reduced product that forms (often an oxide) does not dissolve into the electrolyte, or fall away from the electrode, but forms a film on the surface of the electrode instead. This can significantly impede the reaction as there is reduced electrical contact within the cell. This can substantially prolong the shelf life of the battery, however when the battery discharges the initial voltage may be lower than expected until the coating is broken. This is known as voltage delay.
Battery Death due to Aging
Over the life time of the cell the morphology of the components will change, with effects that are detrimental to the functioning of the cell.
- The crystals in the cell grow larger, increasing the impedance,
- Metallic dendrites grow on the cells causing the electrodes to swell, forcing them closer together and increasing the self-discharge,
- The dendrites may penetrate the separator giving very high self discharge, or even a short circuit.
Eventually, the internal impedance and the self discharge will be so high that the battery is no longer usable.
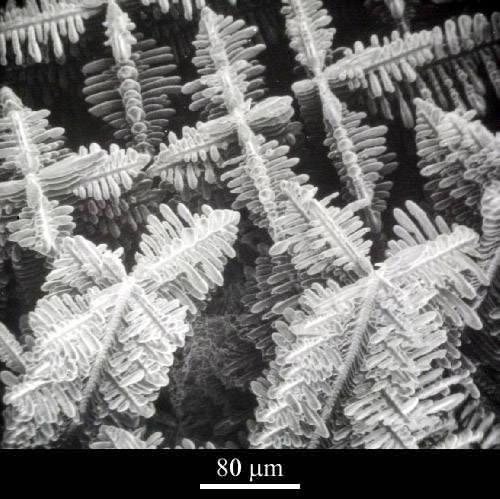
Metallic dendrites (Click micrograph for details)
Physical requirements
This includes the geometry of the cell, its size, weight and shape and the location of the terminals.
Charge/Discharge cycle
There are many aspects of the cycle that need consideration, such as:
- Voltage necessary to charge
- Time necessary to charge
- Availability of charging source
- Potential safety hazards during charge/discharge
Cycle life
The cycle life of a rechargeable battery is the number of discharge/charge cycles it can undergo before its capacity falls to 80%.
Cost
This includes the initial cost of the battery itself as well as the cost of charging and maintaining the battery.
Ability to deep discharge
There is a logarithmic relationship between the depth of discharge and the life of a battery, thus the life of a battery can be significantly increased if it is not fully discharged; for example, a mobile phone battery will last 5-6 times longer if it is only discharged 80% before recharging.
Special deep discharge batteries are available for applications where this might be necessary.
Nickel-cadmium batteries
The exception to this is the nickel-cadmium battery, as subjecting them to partial discharge introduces “memory effects;” the battery appears to “remember” how much charge is being used, and will only recharge that amount rather than the full amount of charge.
In fact the repeated shallow charging causes the crystalline structure in the battery to change: the crystals in the cell grow larger, increasing the impedance and thus reducing its capacity.
Application requirements
The battery must be sufficient for the intended application. This means that it must be able to produce the right current with the right voltage. It must have sufficient capacity, energy and power. It should also not exceed the requirements of the application by too much, since this is likely to result in unnecessary cost; it must give sufficient performance for the lowest possible price.