31.20: Case Study: Fuelling Practicalities
- Page ID
- 33003
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Supplying the cell’s anodes with a constant and consistent supply of hydrogen is no small task. The following diagram shows some ways this can be achieved:
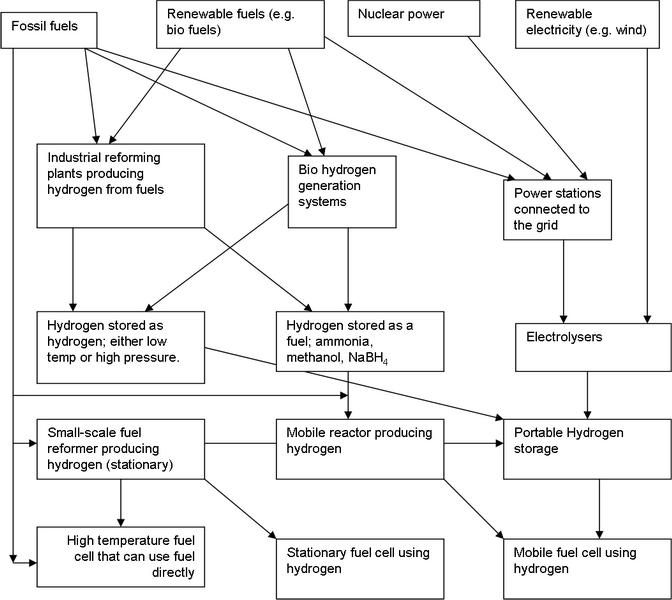
This diagram should be considered it in terms of three levels; where we obtain the energy, how it is used to make hydrogen, and how that hydrogen is stored and used at the cell.
Crude oil derived fuels still account for more than half of the world’s total energy supply (petrol, diesel, aviation fuel, kerosene). These consist of simple and aromatic hydrocarbons of varying length. Add to this the consumption of coal and natural gases and the fossil fuel contribution to global energy supply goes well above 80%. Fossil fuels can either be utilised directly in some high temperature fuel cells, or reformed to hydrogen (see notes on reformers), or used in conventional power plants to produce electricity, which could then power electrolysers.
Renewable fuels are useful bio-matters, such as wood or plants, and renewable gas sources such as the methane produced as waste in landfill sites decomposes. These can again be used to make hydrogen by reforming, bio-generation or by conventional power plants and electrolysers. Nuclear power can power electrolysers to make hydrogen indirectly. The animation below summarises the above:
Fuel storage
Once we have the hydrogen, there are several ways in which it can be stored:
- Compression in gas cylinders
- Cryogenic liquid
- A metal absorber as a metal hydride
- Potentially in carbon nano-fibres (still under development)
- Glass micro-spheres
The specific energy of pure hydrogen (the energy per kilo) is higher than any other fuel at about 120 MJ kg-1. However, its energy density (energy per m3) is very low.
|
Form of storage |
Energy density by weight |
Energy density by volume |
|---|---|---|
|
Gas (20 MPa) |
33.3 |
0.53 |
|
Gas (24.8 MPa) |
33.3 |
0.64 |
|
Gas (30 MPa) |
33.3 |
0.75 |
|
Cryogenic liquid (-253 °C) |
33.3 |
2.36 |
|
Metal hydride |
0.58 |
3.18 |
It’s difficult to get a large mass of hydrogen into a small volume. Whilst fossil fuels can be processed to provide an on-demand supply of hydrogen, or even utilised directly by some cells, there are many applications for which a pure hydrogen supply is the only viable solution. These applications might include anywhere where there is limited space for reforming equipment or where it would be too costly, where emissions must be tightly controlled perhaps or where adding the additional fuel processing equipment would unacceptably reduce the efficiency of the system. Electrical energy output from wind turbines and hydroelectric generators might also be stored as hydrogen.
The safe storage of pure hydrogen presents a tempting challenge to engineers and material scientists. Either very high pressure must be used to squeeze the required mass into a required volume, or very low temperatures used to liquefy it. Hydrogen however, does not liquefy above 22K and even in its liquid phase has rather a low density of 71 kg m-3.
Ways of storing pure H2
|
Method: |
Pros: |
Cons: |
|---|---|---|
|
High-pressure storage of gas. Hydrogen gas can be stored at pressures of up to 30 MPa (300 atm). Composite tanks can be used with aluminium liners and a Kevlar-epoxy shell. |
|
|
|
Liquid hydrogen (LH2) stored at 22 K in thermally insulated tanks, usually like large vacuum flasks. State of the art tanks cool the 22 K LH2 with liquid air at 78 K. |
|
|
|
Metal hydrides |
|
|
|
Glass micro-spheres can be encouraged to absorb H2 gas at higher temperature, then retain it at lower temperatures before releasing it again when the temperature is raised. A technology yet to be proven. |
|
|
|
Carbon nano-fibres
Structure of carbon nano-fibres |
|
|