3.2: Ideal gas and ideal gas equation of state
- Page ID
- 88834
Consider a container of fixed volume filled with a gas. When the container is heated, the gas temperature will increase, causing the gas pressure to increase. The variations of gas pressure and temperature are governed by the equations of state. An equation of state (EOS) is an expression that relates pressure, temperature, and specific volume of a gas.
The simplest equation of state is the ideal gas equation of state, which is expressed as
\[Pv=RT\]
where
- \(m\): mass, in kg
- \(V\): volume, in m3
- \(v\): specific volume, in m3/kg
- \(T\): absolute temperature, in K
- \(P\): pressure, in kPa or Pa
- \(R\): gas constant in kJ/kgK or J/kgK
A gas which obeys the ideal gas EOS is called an ideal gas. The ideal gas model is a hypothetical model. It approximates the 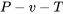 behaviour of a gas at high temperatures and low pressures in the superheated vapour region.
behaviour of a gas at high temperatures and low pressures in the superheated vapour region.
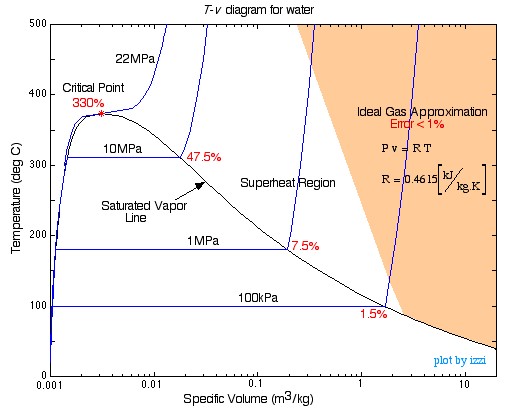
When a gas is at a state near the saturation region or its critical point, the gas behaviour deviates from the ideal gas model significantly. For example, Figure 3.1.1 shows the 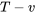 diagram for water. Steam in the shaded region is either at a high temperature or a low pressure. The ideal gas model is valid in this region with a relative error of less than 1%. Moving out of the shaded region and towards the saturated vapour line or the critical point, the relative error increases significantly because the ideal gas EOS can no longer represent the gas behaviour in these regions.
diagram for water. Steam in the shaded region is either at a high temperature or a low pressure. The ideal gas model is valid in this region with a relative error of less than 1%. Moving out of the shaded region and towards the saturated vapour line or the critical point, the relative error increases significantly because the ideal gas EOS can no longer represent the gas behaviour in these regions.
A common mistake that students tend to make is to use the ideal gas EOS in all calculations without evaluating its suitability for the given conditions. It is important to note that, although many gasses may be treated as ideal gases in a certain range of pressures and temperatures, the ideal gas EOS is NOT valid for gases in all conditions. Therefore, it cannot be used without verification. The compressibility factor in Section 3.2 explains how to verify if a gas is an “ideal” or real gas.
Two tanks contain methane. For the given conditions, methane can be treated as an ideal gas.
- Tank 1 has a volume of 0.3 m3, and is at a temperature of 20°C and a pressure of 300 kPa.
- Tank 2 contains 1.5 kg of methane, and is at a temperature of 30°C and a pressure of 800 kPa.
The partition between the two tanks is removed to allow methane in the tanks to mix and reach equilibrium. What is the equilibrium pressure if the temperature of the two tanks is 25°C at equilibrium?

Solution
Methane is treated as an ideal gas at the given conditions.
From Table G1: R=0.5182 kJ/kgK for methane.
Apply the ideal gas law 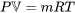 to both initial and final conditions of methane in the two tanks.
to both initial and final conditions of methane in the two tanks.
Tank 1 at the initial condition:
\[P_1\mathbb{V}_1=m_1RT_1\]
\[m_1 =\displaystyle\frac{P_1\mathbb{V}_1}{RT_1 }= \frac{300 \times 0.3}{0.5182 \times (273.15 + 20)}= 0.5925\ \rm{kg}\]
Tank 2 at the initial condition:
\[P_2\mathbb{V}_2=m_2RT_2\]
\[\mathbb{V}_2 =\displaystyle\frac{m_2RT_2}{ P_2}= \frac{1.5 \times 0.5182 \times (273.15 + 30)}{800}= 0.2945\ \rm{m^3}\]
The two tanks are in equilibrium at the final state.
\[m_3 = m_1 + m_2 = 0.5925 + 1.5 = 2.0925 \ \rm{kg}\]
\[\mathbb{V}_3 = \mathbb{V}_1 + \mathbb{V}_2 = 0.3 + 0.2945 = 0.5945 \ \rm{m^3}\]
\[P_3\mathbb{V}_3=m_3R_3T_3\]
\[P_3=\displaystyle\frac{m_3RT_3}{\mathbb{V}_3 }= \displaystyle\frac{2.0925 \times 0.5182 \times (273.15 + 25)}{0.5925}= 543.8 \ \rm{kPa}\]
The equilibrium pressure of the two tanks at the final state is 543.8 kPa.
Important note:
- The temperature must be expressed in Kelvin when applying the ideal gas EOS.
Consider 1 kg of oxygen in a piston-cylinder device undergoing a thermodynamic cycle consisting of three processes.
- Process 1
 2: isochoric
2: isochoric - Process 2
 3: isothermal expansion
3: isothermal expansion - Process 3
 1: isobaric compression
1: isobaric compression
At state 1, T1= 300 K, P1=1.5 atm. At state 2, P2= 3 atm. Treat oxygen as an ideal gas at the given conditions.
- Sketch the cycle on a
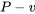 diagram.
diagram. - Determine the temperature, T2 , at state 2, and the specific volume, v3, at state 3.
Solution
The cycle on a 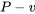 diagram
diagram
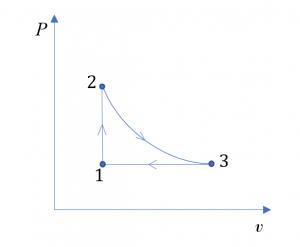
2. Oxygen is treated as an ideal gas at the given conditions.
From Table G1: R=0.2598 kJ/kgK for oxygen.
Apply the ideal gas law 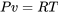 to the three processes.
to the three processes.
Process 1 2 is an isochoric process; therefore, the specific volume remains constant in the process,
2 is an isochoric process; therefore, the specific volume remains constant in the process, 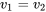
\[\because Pv = RT\]
\[\therefore v_1 = \displaystyle\frac{RT_1}{P_1} \]
\[\because v_1 = v_2\]
\[\therefore\displaystyle\frac{T_2}{T_1} = \displaystyle\frac{P_2}{P_1}\]
\[\therefore T_2 = T_1 \times \displaystyle\frac{P_2}{P_1} = 300 \times \frac{3}{1.5} = 600 \ \rm{K}\]
Process 2 3 is an isothermal expansion process; therefore,
3 is an isothermal expansion process; therefore, 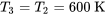 .
.
Process 3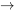 1 is an isobaric compression process; therefore,
1 is an isobaric compression process; therefore,
\[P_3 = P_1 = 1.5 \ \rm{atm}=1.5 \times 101.325=152 \ \rm{kPa}\]
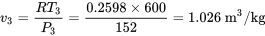
The temperature at state 2 is 600 K and the specific volume at state 3 is 1.026 m3/kg.
Important note:
- The temperature must be expressed in Kelvin when applying the ideal gas EOS.
Query \(\PageIndex{1}\)
Media Attributions
- T-v diagram for water © Israel Urieli adapted by DIANA BAIRAKTAROVA is licensed under a CC BY-SA (Attribution ShareAlike) license


