4.6: Chapter review
- Page ID
- 88843
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Energy can be transferred to and from a closed system by two mechanisms: heat transfer and work. Both heat transfer and work have a significant effect on the total energy as well as the internal energy stored in a system, as expressed in the first law of thermodynamics.
When applying the first law of thermodynamics to a closed system, it is important to evaluate the internal energy of the system at various states of a process, and the boundary work and heat transfer during the process.
- The internal energy is a property of a system. It is a state function and is independent of the process path. In general, the specific internal energy can be found by using thermodynamic tables. For ideal gases, solids or liquids, the specific internal energy can be calculated by using the constant-volume specific heat.
- Heat transfer and boundary work are not properties of a system. They are boundary phenomena and path functions. Their magnitudes depend on the initial and final states as well as the path of a process.
- The boundary work and specific boundary work in a process can be expressed graphically as the area under the process curve in the
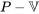 and
and 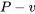 diagrams, respectively; therefore, these diagrams are often used when evaluating the boundary work in a process.
diagrams, respectively; therefore, these diagrams are often used when evaluating the boundary work in a process.


