6.8: Specific entropy of a state
- Page ID
- 88858
6.7.1 Determining the specific entropy of pure substances by using thermodynamic tables
The specific entropy of a pure substance can be found from thermodynamic tables if the tables are available. The procedures are explained in Section 2.4. In addition to the 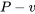 and
and 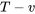 diagrams, the
diagrams, the 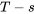 diagram is commonly used to illustrate the relation between temperature and specific entropy of a pure substance. Figure 6.7.1 shows the
diagram is commonly used to illustrate the relation between temperature and specific entropy of a pure substance. Figure 6.7.1 shows the 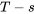 diagram for water.
diagram for water.

Example 1
Fill in the table.
| Substance | T, oC | P, kPa | v, m3/kg | Quality x | s, kJ/kg-K | Phase |
| Water | 250 | 0.02 | ||||
| R134a | -2 | 100 |
Solution:
Water: T = 250 oC, 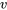 = 0.2 m3/kg
= 0.2 m3/kg
From Table A1: T = 250 oC, 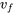 = 0.001252 m3/kg,
= 0.001252 m3/kg, 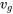 = 0.050083 m3/kg
= 0.050083 m3/kg
Since  , water at the given state is a two phase mixture; the saturation pressure is Psat = 3976.17 kPa, and
, water at the given state is a two phase mixture; the saturation pressure is Psat = 3976.17 kPa, and 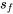 = 2.7935 kJ/kgK,
= 2.7935 kJ/kgK, 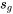 = 6.0721 kJ/kgK
= 6.0721 kJ/kgK
The quality is
\[x = \dfrac{v - v_f}{v_{g} - v_{f}} = \dfrac{0.02 - 0.001252}{0.050083 - 0.001252} = 0.383936\]
The specific entropy is
R134a: T = -2 oC, P = 100 kPa
From Table C1: by examining the saturation pressures at 0 oC and – 5 oC, we can estimate that the saturation pressure for T = -2 oC is about 270 kPa; therefore, R134a at the given state is a superheated vapour.
From Table C2,
\[s\]
\[s\]
\[s\]
\[\because \dfrac{v - 0.207433}{0.2160303 - 0.207433} = \dfrac{s - 1.7986}{1.8288 - 1.7986} = \dfrac{-2 - (-10)}{0 - (-10)}\]
 and
and 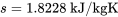
In summary,
| Substance | T
oC |
P
kPa |
v
m3/kg |
Quality x | s
kJ/kg-K |
Phase |
| Water | 250 | 3976.17 | 0.02 | 0.383936 | 4.0523 | two-phase |
| R134a | -2 | 100 | 0.214529 | n.a. | 1.8228 | superheated vapour |
Example 2
A rigid tank contains 3 kg of R134a initially at 0oC, 200 kPa. R134a is now cooled until its temperature drops to -20oC. Determine the change in entropy, 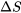 , of R134a during this process. Is
, of R134a during this process. Is 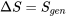 ?
?
Solution:
The initial state is at T1 = 0oC and P1 =200 kPa. From Table C2 in Appendix C,
\[s_1\]
The tank is rigid; therefore, 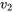 =
= 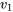 = 0.104811 m3/kg.
= 0.104811 m3/kg.
From Table C1, at T2 = -20oC:
\[v_f\]
\[s_f\]
Because  , the final state is a two-phase mixture.
, the final state is a two-phase mixture.
\[x_2 = \dfrac{v_2 - v_f}{v_g-v_f} = \dfrac{0.104811 - 0.000736}{0.147395-0.000736}=0.70964\]
The total entropy change is
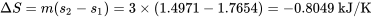
It is important to note that 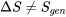 in general. The total entropy of R134a decreases in this cooling process, but the entropy generation is always greater than zero in a real process.
in general. The total entropy of R134a decreases in this cooling process, but the entropy generation is always greater than zero in a real process.
6.7.2 Determining the specific entropy of solids and liquids
The specific entropy of a solid or a liquid depends mainly on the temperature. The change of specific entropy in a process from states 1 to 2 can be calculated as,
\[s_2-s_1=C_pln\dfrac{T_2}{T_1}\]
where
\[s\]
\[C_p\]
\[T\]
6.7.3 Determining the specific entropy of ideal gases
The specific entropy of an ideal gas is a function of both temperature and pressure. Here we will introduce a simplified method for calculating the change of the specific entropy of an ideal gas in a process by assuming constant specific heats. This method is reasonably accurate for a process undergoing a small temperature change.
\[s_2-s_1=C_pln\displaystyle\frac{T_2}{T_1}-Rln\frac{P_2}{P_1}\]
\[s_2-s_1=C_vln\displaystyle\frac{T_2}{T_1}+Rln\frac{v_2}{v_1}\]
where
\[C_p\]
\[T\]
\[P\]
\[s\]
\[v\]
Example 3
Air is compressed from an initial state of 100 kPa, 27oC to a final state of 600 kPa, 67oC. Treat air as an ideal gas. Calculate the change of specific entropy, 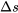 , in this process. Is
, in this process. Is 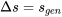 ?
?
Solution:
From Table G1: Cp = 1.005 kJ/kgK, R = 0.287 kJ/kgK
It is important to note that 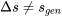 in general. The specific entropy decreases in this process, but the rate of entropy generation is always greater than zero in a real process.
in general. The specific entropy decreases in this process, but the rate of entropy generation is always greater than zero in a real process.
6.7.4 Isentropic relations for an ideal gas
If a process is reversible and adiabatic, it is called an isentropic process and its entropy remains constant. An isentropic process is an idealized process. It is commonly used as a basis for evaluating real processes. The concept of isentropic applies to all substances including ideal gases. The following isentropic relations, however, are ONLY valid for ideal gases.
\[Pv^k= \rm{constant}\]
where
\[k=\dfrac{C_p}{C_v}\]
\[T\]
\[P\]
\[v\]
It is noted that the isentropic relation 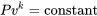 for ideal gases is actually a special case of the polytropic relation
for ideal gases is actually a special case of the polytropic relation 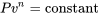 with
with 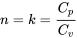 .
.
Example 4
Derive the isentropic relation 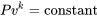
Solution:
For an ideal gas undergoing an isentropic process,
\[\Delta s = s_2 - s_1 =C_pln\displaystyle\frac{T_2}{T_1}-Rln\frac{P_2}{P_1}=0\]
Substitute 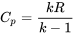 in the above equation and rearrange,
in the above equation and rearrange,
\[\because \dfrac{k}{k-1}ln\dfrac{T_2}{T_1} = ln\dfrac{P_2}{P_1}\]
\[\therefore ln\left(\dfrac{T_2}{T_1}\right)^{\dfrac{k}{k-1}} = ln\dfrac{P_2}{P_1}\]
\[\therefore \dfrac{P_2}{P_1} = \left(\dfrac{T_2}{T_1}\right)^{\dfrac{k}{k-1}}\]
Combine with the ideal gas law, 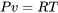 ,
,
\[\therefore \dfrac{P_2}{P_1} = \left(\dfrac{T_2}{T_1}\right)^{\dfrac{k}{k-1}} = \left(\dfrac{P_{2}v_{2}}{P_{1}v_{1}}\right)^{\dfrac{k}{k-1}}\]
\[\therefore \dfrac{P_2}{P_1} = \left(\dfrac{v_1}{v_2}\right)^k\]
\[\therefore Pv^k = \rm{constant}\]
Query \(\PageIndex{1}\)
Media Attributions
- T-s diagram for water © Kaboldy is licensed under a CC BY-SA (Attribution ShareAlike) license


