6.7: The second law of thermodynamics for closed systems
- Page ID
- 88857
The change of entropy in a system is caused by entropy transfer and entropy generation.
\[\Delta \rm{entropy = + in - out + gen}\]
Entropy can be transferred to a system via two mechanisms: (1) heat transfer and (2) mass transfer. It is noted that work is a form of energy transfer; it does NOT contribute to entropy transfer!
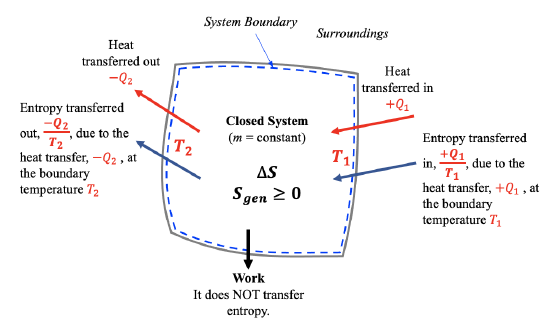
For a closed system, entropy is transferred only by heat transfer, see Figure 6.6.1; therefore, the second law of thermodynamics for a closed system undergoing a process from states 1 to 2 can be written as
\[S_2-S_1=\displaystyle \int_{1}^{2}\displaystyle\frac{\delta Q}{T}+S_{gen}\cong\sum\frac{Q_k}{T_k}+S_{gen}\ \ \ \ \ (S_{gen}\geq0)\]
This equation is also referred to as the entropy balance equation for closed systems. The integral 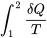 represents the entropy transfer caused by the heat transfer between the system and its surroundings (i.e., heat source and heat sink). Because this integral is difficult to calculate, a common practice is to approximate it with
represents the entropy transfer caused by the heat transfer between the system and its surroundings (i.e., heat source and heat sink). Because this integral is difficult to calculate, a common practice is to approximate it with 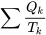 , where
, where 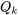 is the heat transfer that takes place at location
is the heat transfer that takes place at location 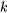 of the system boundary, which has a constant temperature of
of the system boundary, which has a constant temperature of 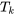 . If heat transfer occurs in multiple locations, all locations must be considered. Attention should be paid to
. If heat transfer occurs in multiple locations, all locations must be considered. Attention should be paid to 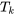 , which represents the temperature of the system boundary or the surroundings if the boundary and the surroundings are in thermal equilibrium.
, which represents the temperature of the system boundary or the surroundings if the boundary and the surroundings are in thermal equilibrium. 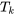 is NOT the temperature of the system itself!
is NOT the temperature of the system itself!
\[T_k = T_{reserviour}\]
\[T_k \ne T_{system}\]
The entropy balance equation is often used together with the first law of thermodynamics, thermodynamic tables (for real substances), or ideal gas equations (for ideal gases). We will demonstrate the applications of the entropy balance equation in Section 6.8.
Query \(\PageIndex{1}\)


