6.12: Chapter review
- Page ID
- 88862
In this chapter, we have introduced two statements of the second law of thermodynamics including the Kelvin-Planck statement and the Clausius statement, and the ideal model of Carnot cycles. These concepts establish a theoretical foundation for evaluating the best possible performance, in terms of the thermal efficiency or COP, of heat engines, refrigerators, or heat pumps operating between two heat reservoirs.
The second law of thermodynamics can be expressed in terms of entropy generation. Due to the existence of irreversibilities, a real process or cycle always proceeds in the direction that obeys 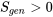 . For an ideal, reversible process or cycle, such as the Carnot cycles,
. For an ideal, reversible process or cycle, such as the Carnot cycles, 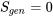 . Any real or ideal process or cycle must satisfy both the first and the second laws of thermodynamics.
. Any real or ideal process or cycle must satisfy both the first and the second laws of thermodynamics.
The first and second laws of thermodynamics are often used together with the thermodynamic tables or ideal gas equations in thermal analysis. When applying the second law of thermodynamics for closed or open systems, it is important to write an appropriate entropy balance equation. Entropy, entropy transfer, and entropy generation are three important concepts in the entropy balance equation. They have different physical meanings.
- Entropy is a measure of the degree of the “randomness” or “disorder” of a system. It is a thermodynamic property of the system and a state function. The change of entropy,
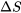 , in a process only depends on the initial and final states of the process.
, in a process only depends on the initial and final states of the process. - Entropy generation,
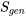 , is a measure of the irreversibilities in a process. It is not a property of the system. It depends on the path of a process; the more irreversible a process is, the larger
, is a measure of the irreversibilities in a process. It is not a property of the system. It depends on the path of a process; the more irreversible a process is, the larger 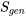 is.
is. - Entropy transfer always accompanies mass transfer and heat transfer in a process or a cycle. Entropy transfer is a boundary phenomenon. It relates to the mass flow rate or heat transfer across the system boundary, as well as the temperature of the boundary.


