6.13: Key equations
- Page ID
- 88863
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
6.12 Key equations
Heat engine
| Net work output | 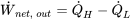 |
| Thermal efficiency of any heat engine | 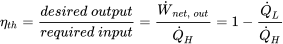 |
| Thermal efficiency of Carnot heat engine | 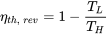
|
Refrigerator and heat pump
| Net work input | 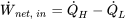 |
| COP of any refrigerator | |
| COP of Carnot refrigerator | 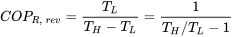 |
| COP of any heat pump | |
| COP of Carnot heat pump | 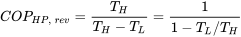 |
Entropy and entropy generation
| The inequality of Clausius | 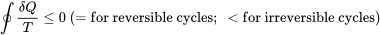 |
| Definition of entropy | 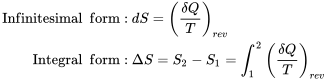 |
| Definition of entropy generation |
The second law of thermodynamics
| For closed systems (control mass) | 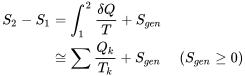 where 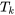 is the absolute temperature of the system boundary, in Kelvin. is the absolute temperature of the system boundary, in Kelvin. |
| For steady-state, steady flow in a control volume (open systems) | 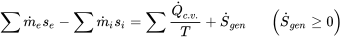 |
| For steady and isentropic flow | 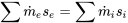 |
| Change of specific entropy between two states of a solid or liquid | 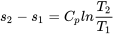 |
| Change of specific entropy between two states of an ideal gas | Assume constant 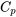 and and 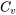 in the temperature range, in the temperature range, 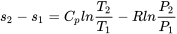
\[s_2-s_1=C_vln\displaystyle\frac{T_2}{T_1}+Rln\frac{v_2}{v_1}\] |
| Isentropic relations for ideal gases | 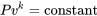
|



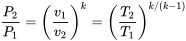
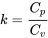 and
and 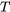 is in Kelvin
is in Kelvin