31.12: Electrolyte
- Page ID
- 32979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
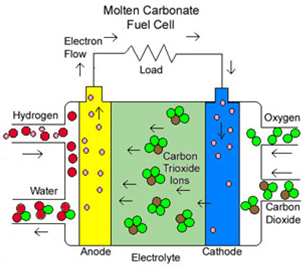
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In most cases, the electrolyte of the MCFC is made of a lithium-potassium carbonate salt heated to 600-1000°C. At this temperature, the salt is in liquid phase and can conduct ions between the two electrodes. The typical mixture ratio of the electrolyte is 62 m/o Li2CO3 and 38 m/o K2CO3 (62/38 Li/K). This particular mixture of carbonate salts melts at 550°C and when it is mixed with lithium aluminate (LiAlO2 is a ceramic matrix retaining the molten salts, it can be used both as an ion-conducting electrolyte and gasketing for the fuel cell stack. Negative carbonate ions (CO32–) are responsible for conduction. As discussed above, the long term performance is an issue for MCFCs.
The following properties of the electrolyte have to be taken into account:
- Volatility of different alkali metal hydroxides generated in the moist cathode atmosphere
- Solubility of the cathode (NiO)
- Segregation of the electrolytes
- Both oxygen and carbon dioxide solubility in the electrolyte
- Oxygen reduction kinetics
Loss of electrolyte mainly occurs at the cathode via hydrolysis:
|
MeCO3 + H2O ⇌ 2MeOH + CO2 |
Especially the electrolyte that has been used unaltered for a long time (since Ketelaar and Broers), Li2CO3/K2CO3 (38/62) eutectic, has a relatively high volatility. This causes the fuel cell to dry out.
The partial pressure due to MeOH varies with the square root of water vapour to carbon dioxide vapour pressures:
\[ p(Me_iOH) = K_i(T) \sqrt{\frac{p_{H_2O}}{p_{CO_2}}} \]
Ki(T) is the equilibrium coefficient of the carbonate ion in the melt according to the equilibrium equation:
|
CO32– + H2O ⇌ CO2 + 2OH– |
The anode off-gas on the other hand can be mixed with air after combustion and reused in the cathode chamber with a sufficiently high air excess. Here oxygen and carbon dioxide are consumed in a ratio of 1:2 (molar) by the cathode process.
The change in composition of the electrolyte due to segregation and the volatility of certain species may result in a change in melting temperature. The electrolyte can solidify, causing the fuel cell to malfunction and sometimes allowing gases to break through. Carbonate ions from the electrolyte are used up in the reactions at the anode, so that we have to compensate for the loss by injecting carbon dioxide at the cathode.
Segregation occurs as the potassium concentration increases near the cathode. This leads to an increased cathode solubility and hence a decline of cell performance.
Recent studies show, that using Na instead of K can decrease the amount of segregation. In a molten binary salt with a common anion, segregation occurs due to the difference in mobilities of the cations. The heavier and bigger potassium and sodium cation is faster lithium in mixtures that have a higher potassium concentration that K2CO3=0.32 (Chemla effect), whereas below this isotachic concentration, lithium is faster.
The partial pressure of carbon dioxide has a much smaller effect on the current voltage curves than the partial pressure of oxygen. Mass transfer limitations are not observed for CO2 but for oxygen at low partial pressures. CO2 transport also hinders the cathode operation much less than oxygen transport since this gas has a much better solubility in carbonate melts than O.
Only the Li2CO3/Na2CO3 eutectic with much lower sodium vapour pressures could assure long term performance of fuel cells. We can conclude that the Li/Na electrolyte is more reliable and safer than Li/K. Since the ionic conductivity of Li/Na carbonate melts is higher than that of Li/K carbonate melts, Li/Na is preferred material as the electrolyte. But since the electrolyte is in the liquid phase, the fuel cell needs a more complex design, compared to other technologies using a solid electrolyte.


