Ferromagnetism
- Page ID
- 331
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Magnetism has been a phenomenon that has captivated humanity for centuries. There are five different types of magnetism, they are diamagnetism, paramagnetism ferromagnetism, antiferromagnetism, and ferrimagnetism. Ferromagnetism being the most common the average person recollects. That is because most people have encountered permanent magnets sometime in their life, and they are made of ferromagnetic material. This is really similar to paramagnetic material but with one important difference that separates them.
Paramagnetism has no net magnetic field because the spins of the electrons are pointing in all different direction. This means that when a strong magnet of either positive or negative attraction is placed near the paramagnetic material a partial alignment of the spins will result in a weak attraction. Where as in ferromagnetism the spins of the electrons are all pointing in the same direction. This is what causes permanent magnets to attract through opposite poles, south to north and vise versa, as well as repel when the same poles are facing each other.
Ferromagnetic Material Usage
The most common ferromagnetic materials are cobalt, iron, nickel, along with Lodestone a naturally magnetized mineral and other rare earth metal compounds. A common usages of ferromagnetic materials affecting our everyday lives is through magnetic storage in the form of data. Otherwise considered non-volatile storage since data cannot be lost when the device it is not powered. An advantage of this storage method is that it is one of the cheaper forms of storing data, as well as having the ability to be re-used. This is all possible because of Hysteresis.
Once ferromagnetic materials are magnetized toward a specific direction it loses the ability to lose its magnetization (Hysteresis). Meaning it will not be able to go back to its original state without any magnetization. But another opposite magnetic field can be applied which would result in the creation of a hysteresis loop, as seen in figure 1. This ultimately is the unique effect that allows these materials to retain data, after the magnetizing field is dropped to zero.

Magnetization of Ferromagnetic Materials
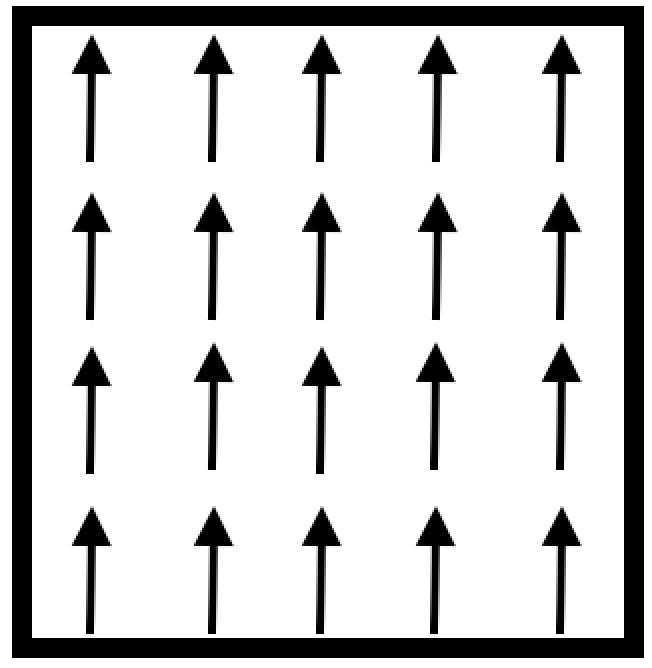
Ferromagnetic materials contain unique magnetic moments that are aligned parallel to each other, all in the same direction (Figure 2). All other types of magnetization have moments in more than one direction. Ferromagnetism is the only magnetization with all same direction moments. Resulting in either attraction or repulsion with other magnetic materials. The north poles attract the south poles, while the same poles repel each other (North to North, South to South). They will have an equal an opposite moments repelling each other. Figure 2 below depicts the magnetic moments in ferromagnetic materials. They experience the same magnitude and they are ordered, without a magnetic field being present.
Classical Explanation
The Weiss theory (Hw), depicts how the Weiss molecular field is proportional to the magnetization of the ferromagnetic material, as shown in the equation below. Where B represents the constant of proportionality.
$$ \ H_\omega = \beta M \label{1} $$
Equation \ref{2} below describes the total magnetic field, with \(H\) as the exterior field.
\[ \ H_{tot} = H + H_\omega = H + \beta M \label{2} \]
Due to similarity with paramagnetism the equation below can be solved and exchanged for \(H\) in the Langevin function.
\[ \ a= \mu_o m_\beta \dfrac{H_(tot)}{ k T} \label{3} \]
\[ \dfrac{M}{M_S} = L ( \dfrac{ \mu_o m_\beta (H + \beta M)}{kT})\label{4} \]
\[ \dfrac{Nm_b}{v} = M_S \label{5} \]
No internal field exists above the Curie temperature \(T_c\), solving Equation \ref{1} gives BM equal to 0. Resulting in the Curie-Weiss law:
\[ \dfrac{M}{H} = \dfrac{ \mu_ o N(m_\beta)^2} {v3k(T-T_c)} = X_F \label{6} \]
Quantum Mechanical Explanation
The quantum mechanical phenomena is the more accurate way to describe the ferromagnetism, since only specific angles of magnetic movement are allowed. Through the classical view point all angles are allowed, because of the Langevin theory making this method extremely unlikely. Therefore, the normalized power law below with a gamma of .5 is an accurate representation of the ferromagnetism phenomena.
\[ \dfrac{M_S(T)} {M_S(T_o)} = \dfrac{T_c - T} {T_c} \label{7} \]
Temperature Dependence
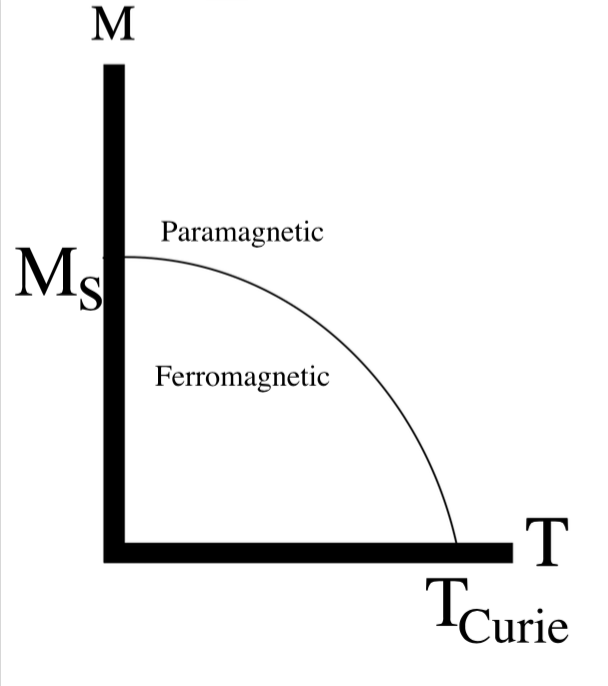
Below the Curie temperature the spins of a ferromagnetic material are of the same magnitude, and well ordered. Once the Curie temperature is reached it means the moments become randomly aligned, meaning that the spin-coupling limit has been surpassed therefore causing the coupling to break down making the material act paramagnetically. Looking at the Figure 3 below, it depicts how the moments are aligned below the Curie temperature (at ferromagnetic) but then above the Currie temperature it becomes paramagnetic. Due to the formation of randomly aligned spins.
Use the section below to provide a short description of the feature, telling the reader what it is used for. The section is also used by other pages to provide an abstract of this page.
Questions
Use the section below to provide a short description of the feature, telling the reader what it is used for. The section is also used by other pages to provide an abstract of this page.
- What are the differences between ferromagnetism and paramagnetism?
- Explain why and how does ferromagnetism behave above and below the Curie?
- What is a common application for ferromagnetism materials and why/how does it work?
Answers
Use the section below to provide a short description of the feature, telling the reader what it is used for. The section is also used by other pages to provide an abstract of this page.
- Ferromagnetic materials has uniform electron spins pointing in the same direction, while paramagnets have spins in all directions. This causes the ferromagnets to have strong attractive or repulsive forces when introduces to a permanent magnet. On the other hand ferromagnets have weak attractions to strong permanent magnets.
- The moments are aligned below the Curie temperature (at ferromagnetic) but then above the Currie temperature it becomes paramagnetic. This is expected because below the Curie temperature the spins are of the same magnitude with order. But then passing the Curie temperature means the moments will become randomly aligned, resulting in coupling breaking down, making the material paramagnetic.
- A common application for ferromagnetic materials is in the use of data storage systems. This is the case because it is cheaper than other methods and the drives can be erased and used again over time. The way that this is possible is because once ferromagnetic materials are magnetized they lose their abilities to demagnetize. Resulting in continues paramagnetic magnetization with or without an exterior source of current.
References
- S. O. Kasap, Principles of Electronic Materials and Devices. McGraw-Hill, 2006
- R. E. Hummel, Electronic Properties of Materials. Springer New York, 2013. p. 347-371
- Ferromagnetism. N.p., n.d. Web. 07 Dec. 2015.
- Encyclopedia Britannica Online. Encyclopedia Britannica, n.d. Web. 06 Dec. 2015.
Use the section below to provide a short description of the feature, telling the reader what it is used for. The section is also used by other pages to provide an abstract of this page.
Contributors and Attributions
- Jose Andrade, Materials Science & Engineering - University of California, Davis
Use the section below to provide a short description of the feature, telling the reader what it is used for. The section is also used by other pages to provide an abstract of this page.

