Chapter 7: Electrochemistry and Corrosion
( \newcommand{\kernel}{\mathrm{null}\,}\)
Over time eventually all materials will degrade. Polymers can be dissolved in organic solvents or crosslinks can be destroyed by UV radiation. Ceramics typically deteriorate in applications where they are utilized at very high temperatures or other extreme environments. And metals will typically undergo corrosion. Corrosion is often the unintentional attach typically at the surface. It is a very significant problem which we typically spend 5% of the GDP each year to prevent corrosion, perform maintenance, or deal with the catastrophic failures due to corrosion. So this is a very stable career choice for Materials Scientists and Mechanical Engineers and one that is very in-demand as the old guard of corrosion engineers are retiring.
What are some examples of corrosion that you have encountered?
There are multiple types of corrosion including:
7.1 Types of Corrosion:
I have recorded a series of lecture videos to supplement this text which can be found in the playlist below:
https://www.youtube.com/playlist?lis...NFEkLqZgWxQhGw
• Uniform Attack
– Occurs uniformly over the entire exposed surface
• Galvanic Corrosion
– Occurs when two different metals are electrically connected and exposed to conducting medium
• Crevice Corrosion
– Concentration difference of ions builds up in the crevices between materials. Very localized corrosion
• Pitting
– Localized corrosion occurring in small pits or holes
• Intergranular Corrosion
– Occurs preferentially along grain boundaries
• Erosion-Corrosion
– Combined chemical attach and mechanical wear
• Stress Corrosion
– Insidious form of corrosion associated with stress corrosion cracking (SCC). Corrosion of a material under applied or residual stress.
• Hydrogen Embrittlement
– Hydrogen induced cracking, H attack
We will cover most of these forms but all of these forms of corrosion are similar in the fact that they involve an electrochemical reaction whereby electrons are lost and gained. These reactions are also called redox reactions which is an abbreviation of reduction and oxidation reactions.
7.2 Electrochemical Nature of Corrosion: Redox Reactions
Corrosion is an electrochemical process where by an element will either gain or lose electrons. During oxidation the element will lose it’s valence electrons. During reduction electrons are gained.
Let’s look at a couple of examples for oxidation:
Fe→Fe2++2e−Al→Al3++3e−
Now how about reduction
2H++2e−→H2Cu2++2e−→Cu
7.3 Electrochemical Cell
These type of reactions which involve only half of the entire redox reaction process are called half cell reactions. They are also called half-cell reactions because they are only describing one half of our typical electrochemical cell which can be seen below:
A couple of key things to note about this diagram. The cathode is defined as positive because it is the electrode where electrons flow in. Theanode is defined as negative as it gives up electrons. Additionally, you can see that cathode is where the reduction reaction occurs and that at the anode the oxidation reaction occurs. In an electrochemical cell or in a redox reaction reduction cannot occur without corresponding oxidation from mass and charge balance consideration. We should also think about the sign of the potential that we measure. If the electrons actually do flow from the anode to the cathode as shown in the figure above the potential should be positive. If the electrons flow in the opposite direction from cathode to anode then we will measure a negative potential and we have mistakenly set up our cell and misidentified the cathode and anode materials.

Let’s take a look at on typical electrochemical cell composed of iron as one of the electrodes and copper for the other. The iron is immersed in a 1M solution containing Fe2+ ions and the copper is immersed in a 1M solution containing Cu2+ ions and it is separated by a membrane as seen below:
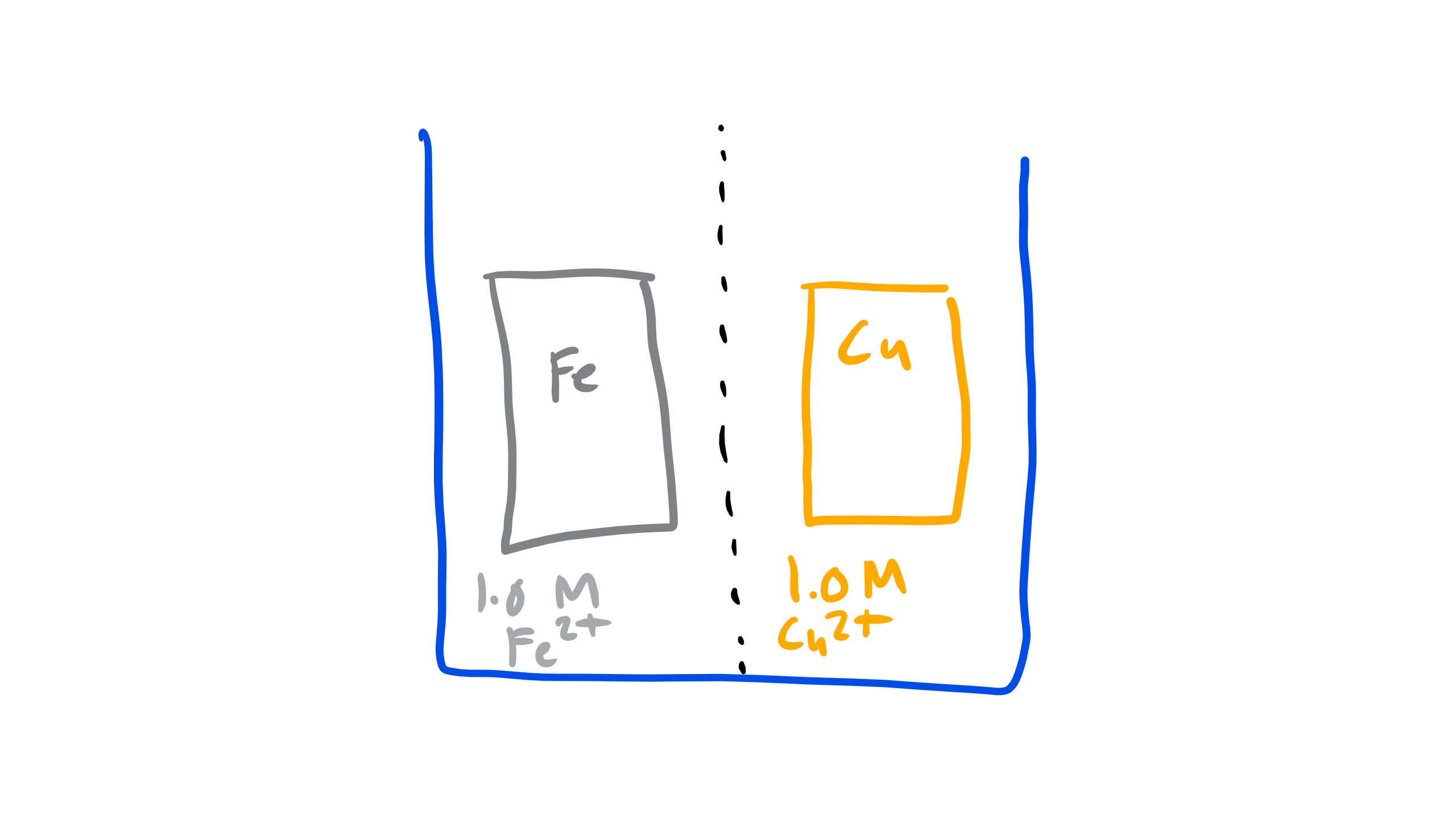
Let’s start simple where will the electrons flow in this cell?
Well the only way that we can find that out is by actually measuring the potential but we can get some idea by looking at the electronegativity of each material. The electronegativity of Cu is 1.9 and the electronegativity of Fe is 1.83. Thus we should expect that Cu should pull electrons from Fe.
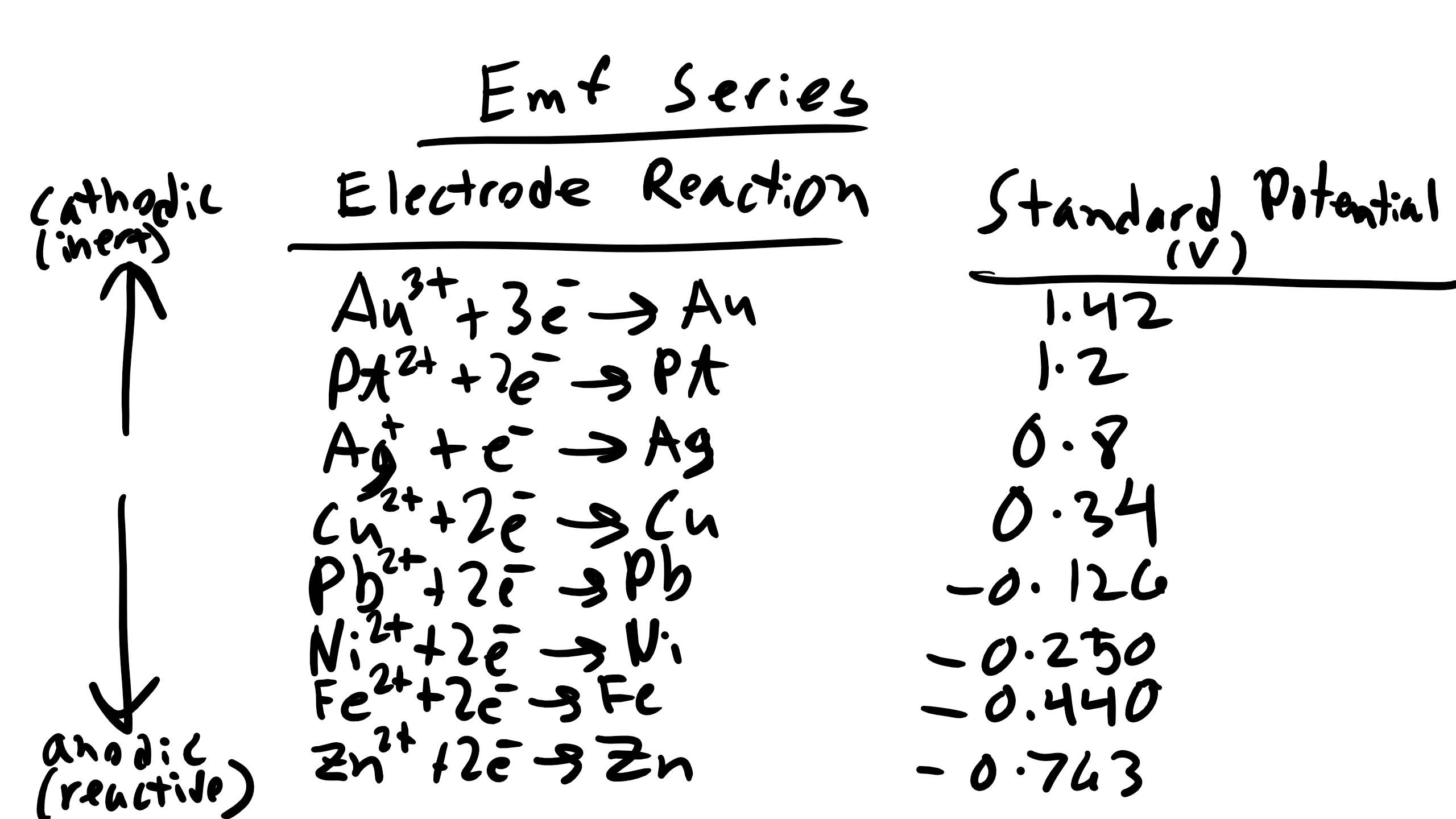
7.4 Standard emf Series:
We can also get an idea about the willingness of a material’s affinity to either give up or gain electrons by looking at a Standard emf Series or a Standard Electrode Potential Series.
You can see that the potential that are at the top of the list are positive and they are materials that are increasingly inert or noble. In other words these materials have a large electronegativity and want to grab electrons. Thus they are typically cathode materials where reduction will take take place. As you move down the list you see that the materials become less noble, more active, and these materials have a very low electronegativity. They want to give away electrons. Note above these are all reduction reactions so the values are assessing in a standard cell how well these materials would fair as cathode materials (positive good, negative bad cathodes should be anodes).
7.5 Half Cell and Full Cell Reactions:
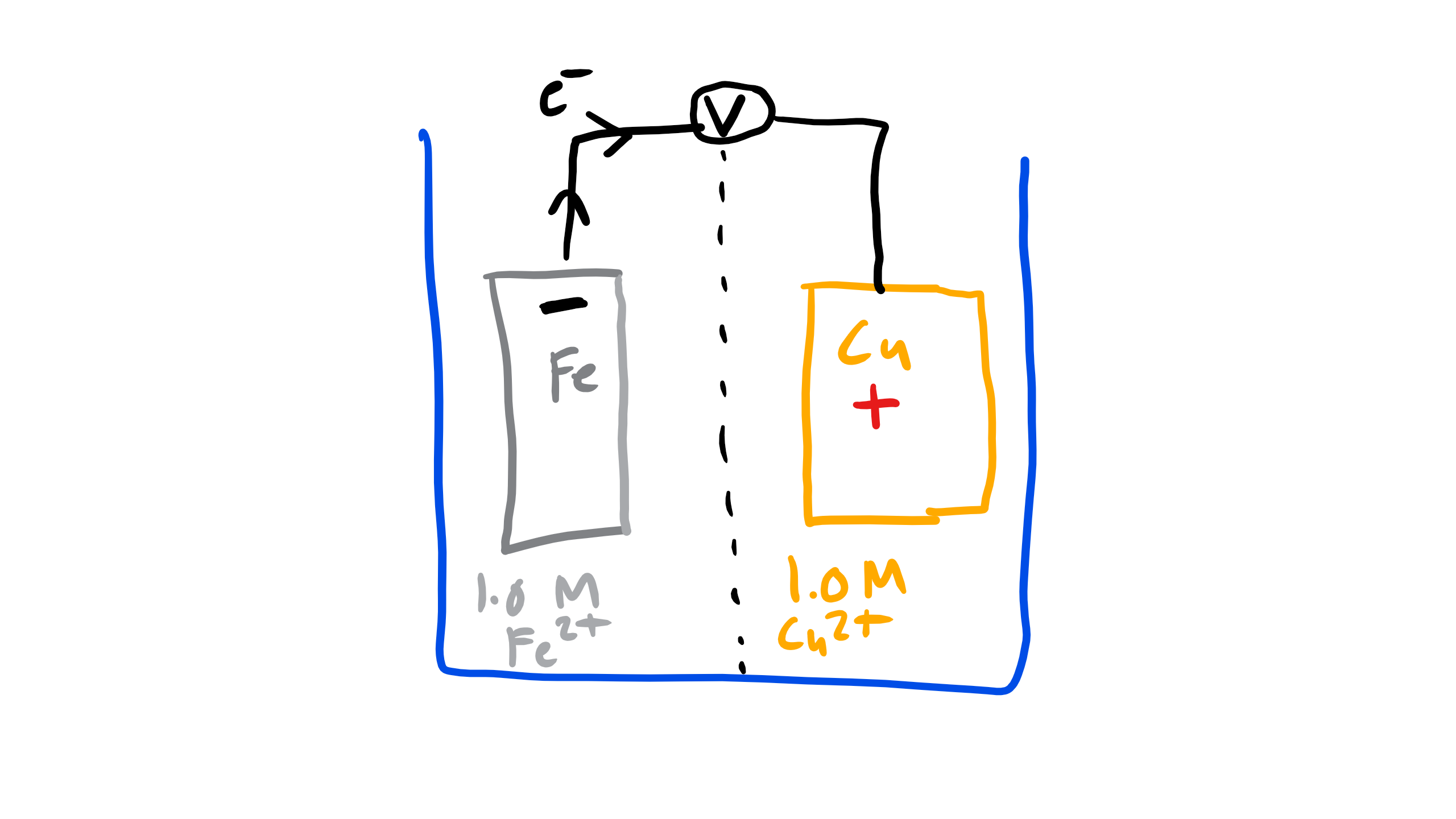
So let’s look back at our old friend the Cu-Fe cell. Let’s assume that from our investigation into electronegativity and looking at the the Standard emf chart that we think that Cu is going to be the cathode where electrons will be gained and reduction will occur and the anode material will be iron where electrons are lost and oxidation occurs[1]. Let’s write these two half cell reaction first at the cathode:
Cu2++2e−→Cu
This half cell reaction will be referred to as the Eocathode.
Now for the anode:
Fe→Fe2++2e−
This half cell reaction will be referred to as the Eoanode. So the full cell reaction will be simple the cathode plus the anode reaction which gives us:
Fe+Cu2+→Cu+Fe2+
The overall potential of the cell will then be given as:
Eocell=Eocathode−Eoanode
where in this case the values of Eocathode = 0.340 according to our table and the value of Eoanode = −0.440. Notice the negative sign here because at the anode we don’t have reduction we have an oxidation reaction. So the overall potential is 0.780V. This value is positive and thus the reaction will proceed spontaneously. If you get a value that is negative then the reaction cannot proceed and you
7.6 What Material Corrodes in Galvanic Corrosion?
You have just done an experiment with a Glavanic electrochemical cell, two metals immersed in a conducting medium and electrically connected. But now you might be asking which material will corrode. Well let’s look at what is happening at the cathode first. Electrons are being pulled to copper and they are going to combine with the Cu2+ ions in the solution and electrodeposit Cu on the surface of the cathode. So the copper electrode will grow essentially. Now on the other side at the anode the Fe is losing electrons and must dissociate and form Fe2− ions in solution so the Fe anode will start to corrode! We can also tell which material in a standard cell configuration will corrode by simply looking at the Standard emf chart and seeing which is more anodic or active, that is the material that will corrode!
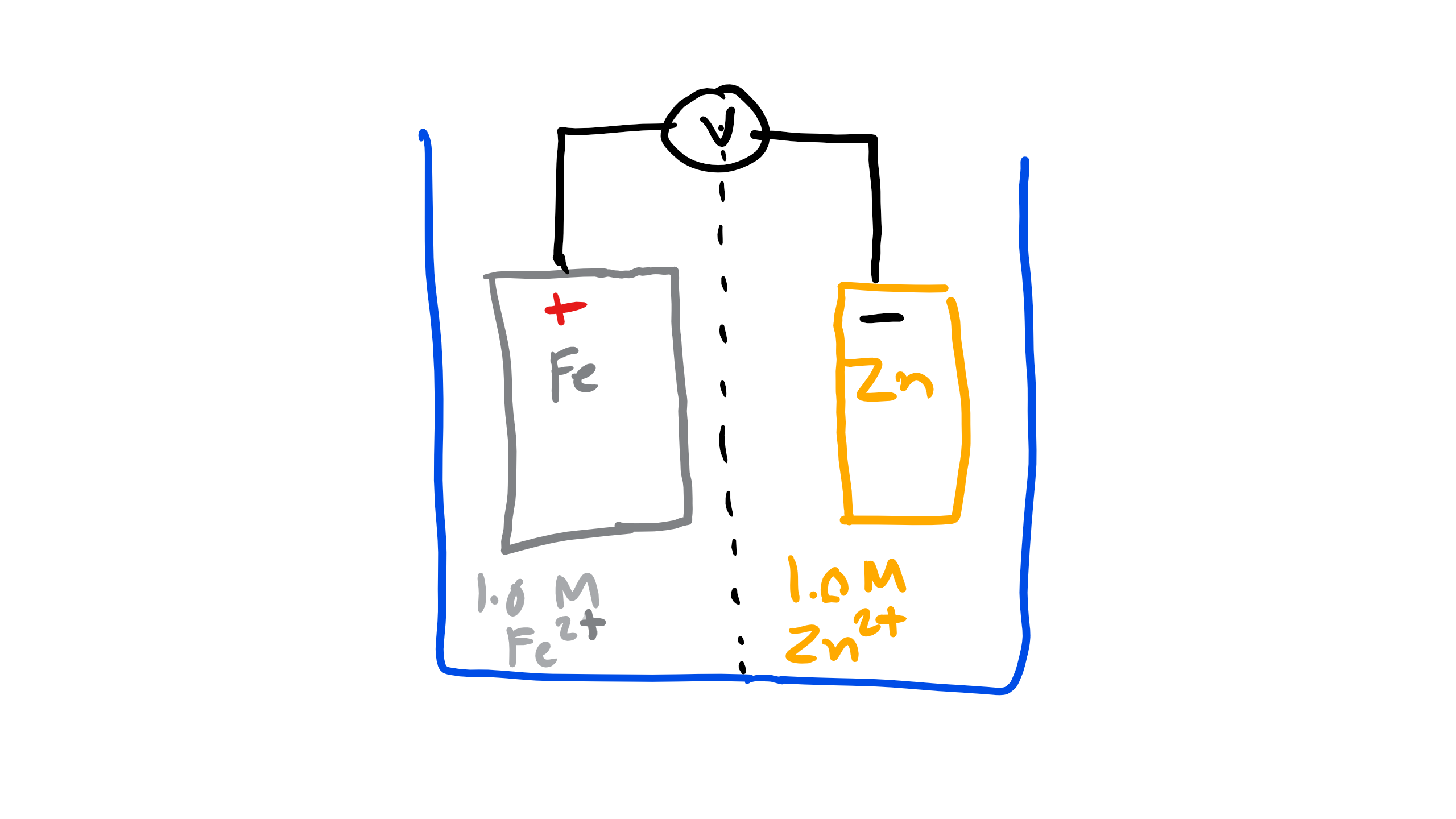
Let’s do another example with Fe-Zn

What is the electronegativity of each material?
Fe is 1.8 and Zn is 1.65.
Which is more noble and which is more active according to the Standard emf?
Fe is more noble and Zn is more active.
Write the half cell reactions
Fe2++2e−→FeZn→Zn2++2e−
Write the full cell reactions
Fe2++Zn→Fe+Zn2+
Calculate the overall potential
Eocell=Eocathode−Eoanode=−0.440+0.763=0.323V
Which material will corrode? Which will electrodeposit?
Zn will corrode and Fe will electrodeposit.
7.7 Nernst Equation:
So far we have been dealing with a very idealized situation, we have always been at room temperature and at 1M solutions. But this is often not the case as temperature and concentrations will typically deviate from this idealized scenario. Luckily we can derive from thermodynamics an equation which describes this change in potential as a function of temperature and activity/concentration. The electrochemical work is defined as:
W=ΔG=QE=−nFE
where Q is charge (Coulomb), n is the number of moles of electrons exchanged in a chemical reaction, F is the Faraday Constant (F = Nave) and is 96485 Cmol, and E is the cell voltage. At standard conditions, the conditions we have been working with up to this point, the change in Gibbs free energy is
ΔGo=−nFEo
Now in general we have the relation that the change in free energy for a chemical reaction is
ΔG−ΔGo=RTlnΠaproductsΠareactants
where Πaproducts are the arithmetic products and reactants. Or similarly
E−Eo=−RTnFlnΠaproductsΠareactants
or for our Cu-Fe example
E−Eo=−RTnFlnaFe2+aCu2+
This is the Nernst equation which is a function of temperature and the activity/concentration.
7.8 Methods to Protect Against Galvanic Corrosion:
There are some relatively simple and straightforward methods to protect against corrosion which can include adding an insulating layer or a coating layer between two metals to break the metal or conducting contact. Polymers are great materials for this. You should also avoid leaving any pockets or crevices uncoated as these are insidious sites for corrosion, specifically crevice corrosion to occur. One of the more creative ways to avoid this issue of corrosion is to provide cathodic protection using a sacrificial anode.
7.8.1 Cathodic Protection:
This mode of protection will be effective for every type of corrosion we previously discussed. Remember that corrosion will occur when the material gives up electrons so to avoid having our material of interest corrode we have to find another source to give up electrons. This can be done using a sacrificial anode or using an imposed current to force electrons to flow in the desired direction. Let’s talk about the sacrificial anode method first.
7.8.2 Sacrificial Anode
Let’s consider an example of an electrochemical cell where we have Cu as the cathode, Fe as the anode which is also connected to Zn. What will happen in this cell? Well look at the electronegativities of Cu, Fe, and Zn. Cu is the most electronegative, then Fe, then Zn. So Cu will pull electrons from Zn and Fe. Fe is going to give electrons to Cu but also pull electrons from Zn. And Zn will give electrons to both Cu and Fe. So the Cu will note corrode and will electrodeposit. While Fe will give up electrons to Cu it will also gain electrons from Zn so it will not electrodeposit. Additionally remember reactions proceed in the direction where the energy barrier is the lowest so pulling electrons from Zn is easier than Fe.

Finally Zn will corrode as electrons are being pulled away by Fe and Cu. This is the sacrificial anode method of protecting against corrosion because we are adding an anode material that is more active than the material we are trying to protect against corroding and we are sacrificing this new material to die a corrosion death, but for a very good cause.
This technique is commonly done for ships and first discovered by Davy and Faraday in 1824 when they placed iron sacrificial anodes to the copper sheath of the hull below the waterline.
This technique is also used in pipelines as well.
7.8.3 Imposed Current
Another and often more costly methods is to apply a biased or imposed current on your system to prevent a material from corroding. What you do is insert a power supply into your circuit as seen below.
You can increase the applied voltage and bias the flow of electrons towards the material that you are trying to protect from corrosion.


