Energy bands in solids and their calculations
( \newcommand{\kernel}{\mathrm{null}\,}\)
When I started to think about it, I felt that the main problem was to explain how the electrons could sneak by all the ions in a metal. By straight Fourier analysis, I found to my delight that the wave diffracted from the plane wave of free electrons only by a periodic modulation. -F. Bloch
No property of solids varies as widely as their ability to conduct electric current and we can see this by the striking difference in resistivity between a good conductor and a good insulator. The electrical resistivity of a pure metal may be as low as 10-12 ohm-cm at a temperature of 1 K, apart from the possibility of superconductivity. The resistivity of a good insulator may be as high as 1022 ohm-cm. This range of 1032 may be the widest of any common physical property of solids. The existence of electron energy bands in solids makes it possible to understand this remarkable span.
We can begin by considering the energy levels of the individual atoms as they are brought together. When the atoms are far apart, the energy of a particular level is the same for each atom. The electrons of a single free-standing atom occupy atomic orbitals, which form a discrete set of energy levels.The allowed energy levels in an isolated atom are often far apart. For example, in Hydrogen, the lowest possible allowed energy (
As the atoms are brought closer together, each of the energy level for each atom changes because of the influence of the other atom. As a result, the level split into two levels of slightly different energies for the two-atom system as in the case of hydrogen molecule ion
Each molecular level can accommodate at most two electrons, of opposite spins, according to the exclusion principle. The
If we bring three atoms close together, a particular energy level splits into three separate levels of slightly different energies. If several atoms are brought together into a molecule, their atomic orbitals split and produce a number of molecular orbitals proportional to the number of atoms. Maria in her term project

Figure
When a large number of atoms (of order 1023 or more) are brought together to form a solid, the number of orbitals becomes exceedingly large, and the difference in energy between them becomes very small, so the levels may be considered to form continuous bands of energy rather than the discrete energy levels of the atoms in isolation. Within an energy band, energy levels are so numerous as to be a near continuum
The Bloch Theorem
The behavior of an electron in a crystalline solid is determined by studying the appropriate Schrödinger equation. This may be written as
where
where
where the function
The vector k is a quantity related to the momentum of the particle. It is possible to write the solution of Equation (1) as
where
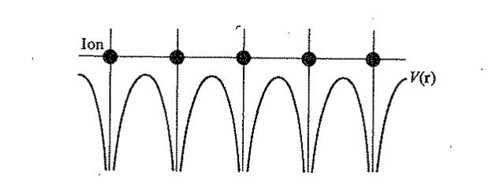
Figure
The only function which satisfies the requirements for all R's is one of the exponential form
- It has the form of a traveling plane wave, as represented by the factor
- Because the electron behaves like a wave of vector
- The Bloch function

Figure
Energy Bands
The energy spectrum result from solving the Equation (1) have large number of solutions, giving discrete energies
The dispersion relation between the energy and momentum of electrons can best be described in reciprocal space. It turns out that for crystalline structures, the dispersion relation of the electrons is periodic, and that the Brillouin zone is the smallest repeating space within this periodic structure. For an infinitely large crystal, if the dispersion relation for an electron is defined throughout the Brillouin zone, then it is defined throughout the entire reciprocal space

Figure
The parabolic representation of the dispersion curve for a free particle,
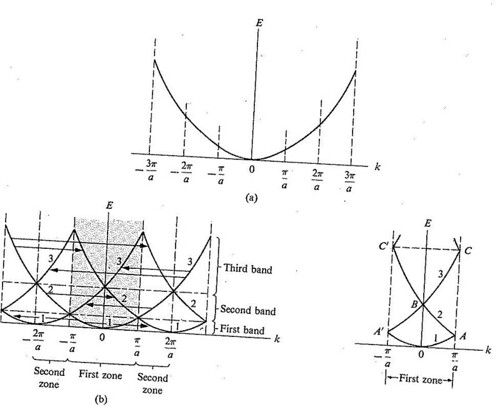
Figure
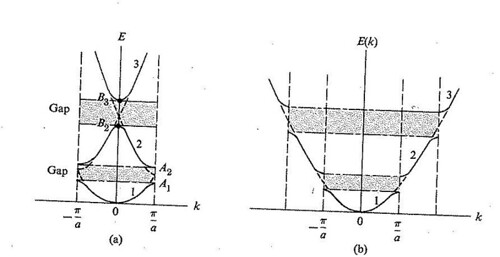
Figure
Any solid has a large number of bands. In theory, it can be said to have infinitely many bands (just as an atom has infinitely many energy levels). However, all but a few lie at energies so high that any electron that reaches those energies escapes from the solid. These bands are usually disregarded. Bands have different widths, based upon the properties of the atomic orbitals from which they arise. Also, allowed bands may overlap, producing (for practical purposes) a single large band. Figure
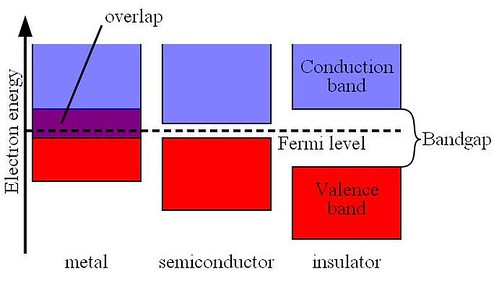
Figure
Metals contain a band that is partly empty and partly filled regardless of temperature. Therefore they have very high conductivity. The lowermost, almost fully occupied band in an insulator or semiconductor is called the valence band by analogy with the valence electrons of individual atoms. The uppermost, almost unoccupied band is called the conduction band because only when electrons are excited to the conduction band can current flow in these materials. The difference between insulators and semiconductors is only that the forbidden band gap between the valence band and conduction band is larger in an insulator, so that fewer electrons are found there and the electrical conductivity is lower. Because one of the main mechanisms for electrons to be excited to the conduction band is due to thermal energy, the conductivity of semiconductors is strongly dependent on the temperature of the material. Fermi energy concept have been discussed by Coons in the term project
The bandgap of a semiconductor is always one of two types, a direct bandgap or an indirect bandgap. The minimal-energy state in the conduction band, and the maximal-energy state in the valence band, are each characterized by a certain
This band gap is one of the most useful aspects of the band structure, as it strongly influences the electrical and optical properties of the material. Electrons can transfer from one band to the other by means of carrier generation and recombination processes. The band gap and defect states created in the band gap by doping can be used to create semiconductor devices such as solar cells, diodes, transistors, laser diodes, and others
References
- Kittel, Charles. “Introduction to Solid State Physics”, Chapter 7. John Wiley & Sons, Inc., (2005).
- Omar, M. Ali. “Elementary Solid State Physics”, Chapter 5. Pearson Education, (2007).
- http://en.Wikipedia.org/wiki/Electro...band_structure
- Hoffmann, R. How chemistry and physics meet in the Solid state, Angew. Chem. Int. Ed. Engl. 26 (1987) 846-878.
- Kohanoff, J. Electronic Structure Calculations for Solids and Molecules, Cambridge University Press, (2006).
Contributors and Attributions
ContribMSE5317

