Plasmon Resonance
- Page ID
- 369
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Plasmon resonance is beginning to receive more recognition in the fields of chemistry, physics, and materials science due to the wide variety of possible applications including but not limited to optical sensing, data storage, light generation, biomedicine, and electronics. Because of their growing popularity, they have become a topic of more research in order to try to understand their properties and how to harness and control their potential uses. This article will discuss what (surface) plasmon resonance is and some applications in which it is currently being used.
What are plasmons?
The classical physics approach can be used to describe plasmons. In this analogy the free electrons in a metal are treated as a liquid, entirely composed of electrons, that has a very high density (plasma). The fluctuations of density that appear on the surface of this material are called plasmons or surface plasmons. Each plasmon represents the quantizations of classically oscillating plasma waves. This means the plasmons represent discreet values of an oscillating plasma wave, therefore most of their properties can be directly derived from Maxwell's equations.
What is surface plasmon resonance (SPR)?
Surface plasmon resonance refers to the electromagnetic response that occurs when plasmons are oscillating with the same frequency on the surface of a material. As these plasmons oscillate at specific resonant frequencies, they move with periodic driving forces that can become large amplitude oscillations when they interact. This phenomenon is stimulated by a light source. The frequency of the incidence of light must be equal to the natrual frequency of the material or resonance will not occur. These oscillations travel on the surface between the material and air and travel in the direction of the negtaive dielectric material surface. Because these plasmons are on this boundary, they are very sensitive to a change in external stimuli such as the absorption of energy into the material. In order to excite the surface plasmons in a manner so that they can resonate, there are two popular configurations used to excited surface plasmon waves currently, the Otto and Kretschmann configurations.
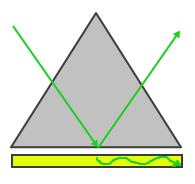 |
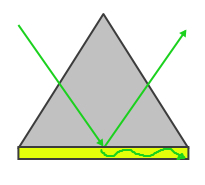 |
| Figure 1: Otto Configuration | Figure 2: Kretschmann Configuration |
The Otto configuration, shown in Figure 1, utilizes a light to illuminate a glass block or prism and the light wave is totally internally reflected and a thin metal film (typically gold or silver) is placed close enough so that the internally reflected light can interact with the plasmons on the surface and excited them. The Kretschmann configuration, shown in Figure 2, is more commonly used and refers to when the thin metal film is evaporated onto the glass block as opposed to being separate. The wave of light passes through the glass block and the metal film, exciting the plasmons on the opposite side of the film.
Surface Plasmon Resonance and Nanoparticles
Nanoparticles are of interest to the scientific community for a multitude of reasons including their large surface area to volume ratio which makes them very reactive to external stimuli quickly, the fact that they operate on a quantum mechanics scale, and because the nanoscale is the level at which many biological processes occur. Understanding how nanoparticles work helps researchers to be able manipulate the properties of nanoparticles for desired outcomes.
One of the important properties of nanoparticles is that they exhibit SPR. When electric fields of light are directed at nanoparticles, the surface plasmons become excited and begin to resonate. This electric field also creates a separation of charge, which can be seen in Figure 3, that then forms a dipole oscillation in the same direction as the electric field of light. Due to the face that the frequencies are the same, the SPR allows a strong abosrption of the incidence light while also allowing some scattering of light; these can be measured using a UV-VIs Spectrometer. The SPR band intensity and wavelength is dependent on the properties of the particle, including the shape, structure, metal type, size, and dielectric material surrounding the medium which can include air. The band intensity is strongest for metals Ag and Au, however, other metals like Cu have been used before. Au is most commonly used to observe this phenomenon due to the fact that it is biologically compatible and inert even though Ag exhibits the strongest bands and sharpest peaks. At the nanoscale, SPR works the most efficiently and is capable of being observed through spectrometry.

This feature of nanoparticles is crucial to being able to "tune" them. It is desirable for scientific research and engineering to be able to control the way a nanoparticle behaves and this can be done by understanding that changing the properties of the nanoparticle will change their behavior. For instance, if nanoparticles are allowed to agglomerate in a solution, becoming larger, their surface plasmons will begin to behave differently, notably the frequency of light that is absorbed by the material will change and therefore the color of the solution of nanoparticles will also change. Extensive research has been directed towards changing the size and shape of Au nanoparticles in an attempt to tune the SPR.
Plasmon Resonance Energy Transfer (PRET) and SPR Imaging
Plasmon Resonance Energy Transfer occurs when nanoparticles are connected to molecular chromophores (an atom or molecule whose presence is responsible for the color of the compound), then the plasmon resonance energy can be transferred to the molcular chromophore. The transfer of this energy paired with the natural frequencies of the biomolecules causes an overlap of resonant energy peak positions. The overlap of these two frequencies can cause spectral quenching dips on the Rayleigh scattering spectrum of a single nanoparticle. This quenching allows for ultrasensitive nanoscopic absorption spectroscopy, which is much more specific as well as faster and more efficient than optical absorption spectroscopy.

This transfer of energy through the material, allows for SPR imaging. A very simple form of this is shown in Figure 4. This demonstrates the basic principle that the energy is transferred to the ligands and biomolecules which in turn, change the amount of reflectivity of light and reflect back a gradient of absorbed spectra that helps to understand the composition of the material. Imaging technology today uses a p-polarized HeNe laser beam as a light source and the reflected light is directed at a CCD camera. By using this camera, 3D images of binding techniques of biomolecules can be observed as well as being able to identify specific versus non-specific adsorption processes, biocharacterizaion, and understanding where the molecule is in space based on the light intensity gradient. This method of imaging is high speed and reactions and binding can be observed in real-time, which helps to better understand the behavior of the molecules.
Surface Plasmon Resonance and Semiconductors
In the past, SPR was limited to metals like Ag, Cu, or Au nanostructures, however, recently there has been a surge in research and data revealing that this property may be a characteristic of any nanoscale structure that carries holes and electrons. Currently, the understanding of charge carrier interactions and dynamics is poorly understood and difficult to observe because of their nanoscale nature. To better understand the properties of these carriers and how they behave within semiconductors could revolutionize semiconductor technologies. With new understandings of how the materials are behaving on a nanoscale comes new ways to manipulate the materials for desired outcomes.
It was discussed earlier in this article that nanoparticles can be "tuned" for properties. Metallic nanoparticles can be tuned for their shape, size, compostition, etc., however, there is one form of tuning that still remains singular to semiconducting materials and that is the ability to tune the free carrier concentration in the material. Ag and Au nanocrystals have a high number of carriers so it is difficult to change their properties by changing carrier concentration. Semiconducting materials have carrier concentrations around 1016-1021/cm3 so the addition of tens of carriers can have an effect on these materials. This smaller scale allows for much more control. In a way, metallic tuning of nanocrystals is static, once tuning of the SPR has occured, it cannot be changed. Semiconducting materials allow for more freedom in this department, one batch of material could be tuned several different times simply by changing the carrier concentration.
By changing the plasmon frequencies of doped semiconductors through the change of the material using carrier concentration, new opportunities arise for plasmonic manipulation of light. Since the plasmon peak can be adjusted based on doping, research has focused on tin-doped indium oxide where it has been shown that the range of absorptions can be shifted to near infrared on the electronmagnetic spectrum. This alone opens up possibilities for controlling optical coupling in and out of nanoplasmonic devices such as thin film semiconductors or tuning the plasmonic enhancement of spectroscopic signatures. It is hopeful that the discovery of this application for SPR will drastically modulate the transmittance of solar infrared radiation.
Questions
- What is the difference between the Otto configuration and the Kretschmann configuration?
- Why are gold nanoparticles more commonly used than silver?
- What types of images can be seen using SPR imaging?
Answers
- The Otto configuration is built with a separation between the glass prism and the thin metal film while in the Kretshmann configuration the gold thin film is evaporated onto the glass.
- Although silver particles provide sharper peaks and stronger bands, gold nanoparticles are not as reactive in biological systems and they are also more biocompatible meaning they have no impact on the system being observed if it is biologically related.
- Through SPR imaging, images of how the light interacted with the molecules can be seen for instance images of how binding occurred, how the material adsorbed, a characterization of what molecule is being observed, and a gradient of light intensity which can show exactly where a molecule is in space.
References
- Biosensing Instruments. "Technical Note 101: Principle of SPR Detection: Intensity Profile and Shift of the SPR Angle | Biosensing Instrument." Biosensing Instrument. Biosensing Instrument, n.d. Web. 09 Dec. 2015.
- Garcia, Guillermo, Evan Runnerstrom, Anna Llordes, Andre Anders, Delia J. Millron, Thomas J. Richardson, Rafaella Buonsanti, and Rueben J. Mendelsberg. "Surface Plasmon Resonance Sensors." Nano Letters (2011): A-F. Lawrence Berkeley Lab. American Chemical Society Publications, 28 July 2011. Web. 8 Dec. 2015.
- HORIBA. "The Principle of Surface Plasmon Resonance Imaging (SPRi)." The Principle of Surface Plasmon Resonance Imaging (SPRi) - HORIBA. HORIBA, 2015. Web. 09 Dec. 2015.
- Huang, Xiaohua, and Mostafa A. El-Sayed. "Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy." Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. Ministry of Science & Technology, Egypt, 12 Feb. 2010. Web. 08 Dec. 2015.
- Long, Yi-Tao, and Chao Jing. Localized Surface Plasmon Resonance Based Biosensors. Shanghai: Springer, 2014. Google Books. Springer, 10 Apr. 2014. Web. 09 Dec. 2015.
- Petryayeva, Eleonora, and Ulrich J. Krull. "Localized Surface Plasmon Resonance: Nanostructures, Bioassays and Biosensing—A Review." Localized Surface Plasmon Resonance: Nanostructures, Bioassays and Biosensing-A Review. Natural Sciences and Engineering Council of Canada, 1 Sept. 2011. Web. 09 Dec. 2015.
- Sigma-Aldrich. "Gold Nanoparticles: Properties and Applications." Sigma-Aldrich. Sigma-Aldrich, n.d. Web. 09 Dec. 2015.
Contributers
- Hannah Kearney (B.S. Materials Science and Engineering, University of California, Davis | June 2016)

