Light Emitting Diodes
- Page ID
- 364
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)LEDs are p-n junction devices made from extrinsic semiconductors. An n-type and a p-type semiconductor are put in contact with each other to form a p-n junction diode. The difference in doping creates a depletion region at the contact interface. In this region there are no stationary charges: an electric field is formed from the opposite charges of holes and electrons attracting each other. majority carriers (electrons from the n-type and holes from the p-type) from each side move through the depletion region.
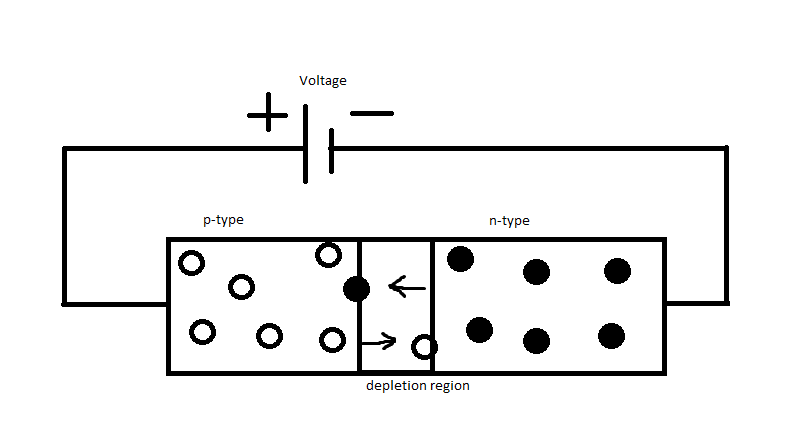
Figure 1 represents how an LED is connected in a circuit. The black dots are the electrons and the circles represent holes. The arrows indicate the direction the majority carriers diffuse across the depletion region. For an LED to function the sign of the terminal from the voltage source should match that of the majority carrier charge. The positive end of the voltage source is connected to the p-type side in which the majority carriers (holes) have a positive charge. This is known as forward bias. As the majority carriers from each side move across to the opposite semiconductor, some electrons and holes collide with each other. In each collision an electron and hole essentially annihilate each other and the energy left over is used to emit a photon.
How LEDs Work
Band-to-band recombination is the main mechanism that describes carrier activity within an LED. When an electron in the conduction band meets a hole in the valence band it falls to a lower energy level (valence band) and releases energy in the form of a photon. The electron drops from band-to-band. When an LED is forward biased, the bands bend slightly in a way to decrease the energy barrier between the n- and p-type semiconductors. This reduced energy barrier enables more of the majority carriers to diffuse to the opposite side of the junction. Since the electrons from the n- side become the minority once they reach the p- side, recombination is more likely to occur.

On the right half of Figure 2 is the n-type semiconductor, shown with electrons (black dots) being the majority carriers. Forward biasing causes the single Fermi level (under equilibrium conditions) to split into two quasi-Fermi levels Fp and Fn. These energy levels are shown as the solid lines near the valence and conduction bands for the p- and n-type semiconductors respectively. The large arrow near the majority electrons represents the direction electrons diffuse across the depletion region. The electrons are represented by the black dots and the holes are the circles. As an electron from the n-type semiconductor passes the depletion region (represented by the diagonal lines) it recombines with a hole in the valence band. This results in the electron losing its energy and falling to the valence band where the hole was, represented by the circle with an 'x.' The energy lost from the electron is conserved and used for the emission of a photon, or particle of light.

When an electron in the conduction band recombines it prefers to go to the highest energy state (the peak) in the valence band because according to semiconductor band gap theory that is where most of the holes are. In direct band gap semiconductors like gallium nitride most of these electrons are in the trough of the conduction band and may move to the valence band without any change in momentum from a phonon. The energy versus momentum plots of Figure 3 show that for an electron to recombine in an indirect semiconductor (silicon for example) additional momentum is required in the form of a phonon. The involvement of a phonon is not likely to occur. It is more efficient that LEDs are made from direct semiconductors so that nothing else (phonons) is required. The point of the device is to create photons from an electron-hole pair and it would be highly inefficient to introduce extra energy for the electron and hole to recombine. For these reasons indirect semiconductors are not likely to be used for LEDs.
Temperature Dependence
In LEDs, the band gaps of the semiconductors increase as temperature decreases. Lower temperatures cause band gaps to increase because the semiconductor's lattice parameter becomes smaller, meaning atoms get closer together. This results in valence electrons getting closer to other valence electrons, increasing electrostatic potential energy \[U=\frac{qQ}{4 \pi \epsilon_0 r}\] where r is the distance between a free electron and a valence electron, \(\epsilon_0\) is the permitivity of free space, and q and Q represent individual charges. r decreases, which increases U. The higher electrostatic potential energy of the electrons creates a higher band gap, as the interactions would make it more difficult for electrons to move to the conduction band. Compare two temperature situations for an LED: at room temperature and after it is dipped in liquid nitrogen. The increase in band gap due to lower temperature would require a higher supply voltage to turn on the LED. This increased band gap energy would be used when an electron recombines with a hole, resulting in a shorter wavelength of light. Conversely if the temperature is increased past room temperature the band gap would become smaller, meaning the semiconductor is becoming more conductive and less voltage would be required to power the LED.
Band Gap and Photon Wavelength Relation
The band gap energy of an LED can be determined by measuring the voltage across an LED at the point which the LED barely begins to turn on. This voltage can be converted to eV by simply multiplying by the charge of one electron (e). For example if the voltage is measured to be 1.7 V, the band gap energy would be 1.7 eV. Since LEDs utilize band-to-band recombination, an electron will drop the full length of the band gap. In other words, the voltage across the LED corresponds to the value of the band gap energy. Energy of the emitted photon is given by \[E=hf=\frac{hc}{\lambda}\] where h is Planck's constant (in units of eV*s), c is the speed of light in vacuum, f is frequency, and \(\lambda\) is the wavelength of the photon.
| LED color | Wavelength Range (nm) | Semiconductors Used |
|---|---|---|
| Red | 625-760 | AlGaAs |
| Orange | 600-625 | GaAsP |
| Yellow | 577-600 | AlGaInP |
| Green | 492-577 | GaN |
| Blue | 455-492 | ZnSe |
| Violet | 390-455 | InGaN |
As listed in Table 1, the color the LED emits depends on the wavelength of the photons emitted from recombination. The type of semiconductor used in the device determines the band gap energy which is used to create photons of different wavelengths. Therefore, the color of the LED is directly material-related.
Questions
- At room temperature, the voltage across an LED was measured to be 1.67 V. What is the band gap of this semiconductor, and what wavelength of light is emitted?
- Why are most LEDs made from direct semiconductors?
- Does an LED work in forward or reverse bias? Why?
Answers
- The band gap is 1.67 eV, which corresponds to a wavelength of 743 nm. Red light is emitted.
- LEDs are mostly made from direct semiconductors because no change in momentum is required for an electron in the conduction band to recombine with a hole in the valence band.
- Forward bias so that the energy barrier between the p- and n-type semiconductors is reduced to allow more of the majority carriers to diffuse to the opposite side of the junction, resulting in more recombination.
References
- Pierret, Robert F. (1996). Semiconductor Device Fundamentals. Reading, Mass.: Addison-Wesley, 1996. Print.
- Umran S. Inan & Aziz S. Inan. (2000) Electromagnetic Waves. Upper Saddle River, New Jersey: Prentice Hall, 2000. Print.
- Fawwaz T. Ulaby & Eric Michielssen, et al. (2010). Fundamentals of Applied Electromagnetics. Upper Saddle River, NJ: Prentice Hall, 2010. Print.
- Hummel, Rolf E. (2012). Electronic Properties of Materials. New York, NY: Springer, 2012. Print.
Contributors and Attributions
- Camron Noorzad, Electronic Materials Engineering UC Davis undergraduate

